MLytics Studio - Analytics Redefined

A Clinical Analytics platform for innovation, productivity and compliance.
Works seamlessly with your current infrastructure, tools, technologies and processes.Guided Auto
Allows a user to search from a growing list of report templates and create a report by simply selecting the required template
Playground
Your playground to explore the complexities of the data and interactively create custom reports, without writing any code
Features
Exploratory Analysis
Helps discover the hidden patterns in the data with automated and guided analytics.
Scalable by design
By leveraging Docker and Kubernetes technologies, the platform is scalable from 10s to 1000s of users.
Simple and intuitive UI
Users with any level of expertise can easily interact with minimal learning curve
Fully compliant and secure
21 CFR part 11 compliance, full support for versioning and audit trail, platform built with a compliance-first mindset.
Project, Team, and workflow management
provides tools and features that let the teams be efficient, agile, and organized.
Services
Obtain deeper insights across your product teams by tapping into Data Sciences, Biostatistics, Clinical Standards and Statistical Programming, and Medical writing expertise from MLytics Life Sciences.
Data Sciences
Our experienced team provides Real-world evidence (RWE), Registries, Healthcare and clinical data analysis and modelling
Biostatistics
We provide statistical study design, data analysis, and interpretation of statistical outcomes.
Clinical Data Standards & Statistical Programming
Our experienced team provides programming services including generation of compliant submission datasets and statistical analyses.
Medical Writing
Our writers work side-by-side with our clinical team and biostatisticians to produce robust protocols, clinical study reports (CSRs), investigational annual reports, regulatory briefing documents and scientific journal publications.
About Us

Our Team
We are a closely-knit team of passionate, motivated, and goal-oriented individuals who are dedicated to making a positive difference in the life sciences and healthcare industry through practical application of cutting edge state-of-the-art technologies.
Our Mission
Our mission is to provide purpose-built solutions, services, and consulting for the development of life science products that contribute to a safer, better world.
Our Vision
Simplify life sciences’ challenges and improve world health through life sciences innovations.What makes it amazing
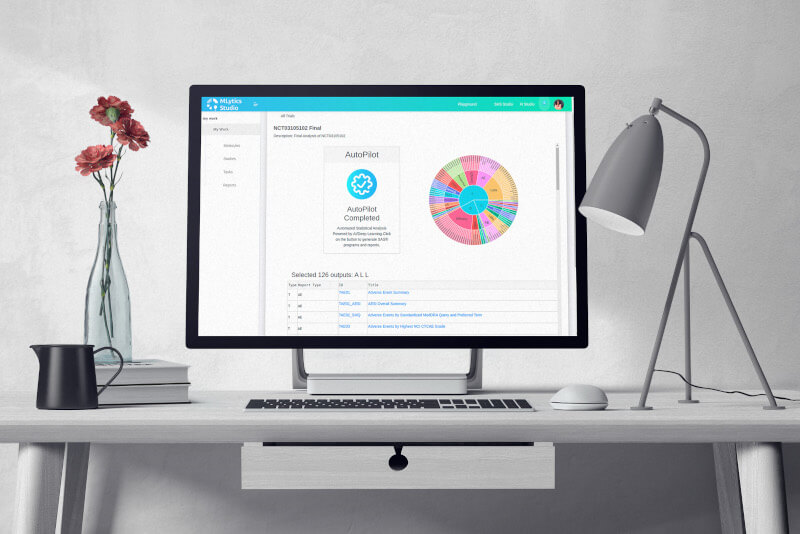
Autopilot
Autopilot, powered by AI/Deep Learning algorithms trained on historical clinical trial data, analyzes Study Protocol, Statistical Analysis Plan, patient data, and metadata to automatically generate SAS® programs, and create reports for a specific target submission task. These algorithms learn on an ongoing basis, build upon the knowledge graphs and keep getting better over time.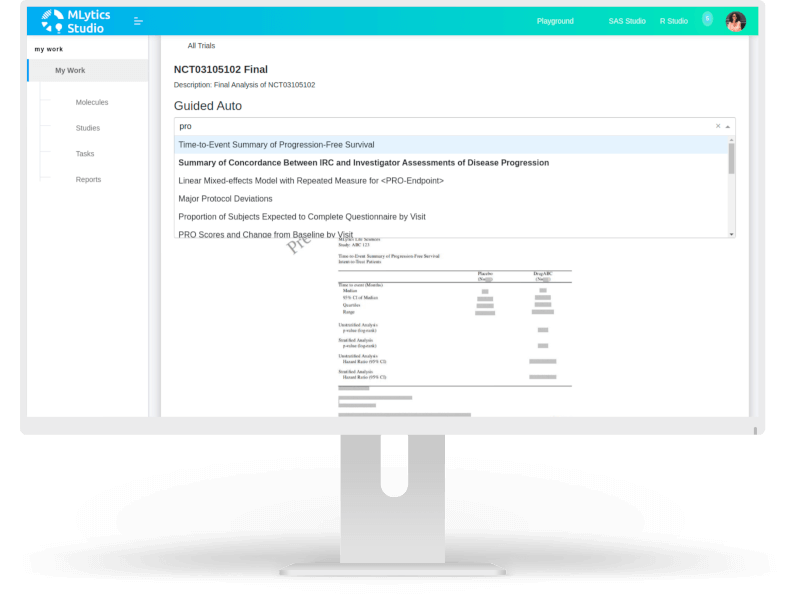
Guided Auto
Allows a user to search from a growing list of report templates and create a report by simply selecting the required template. Submission ready programs are automatically generated. SAS or R skills are optional!

Playground
Your playground to explore the complexities of the data and interactively create custom reports, without writing any code. When you are satisfied with the report, submission ready SAS programs are just a click away!
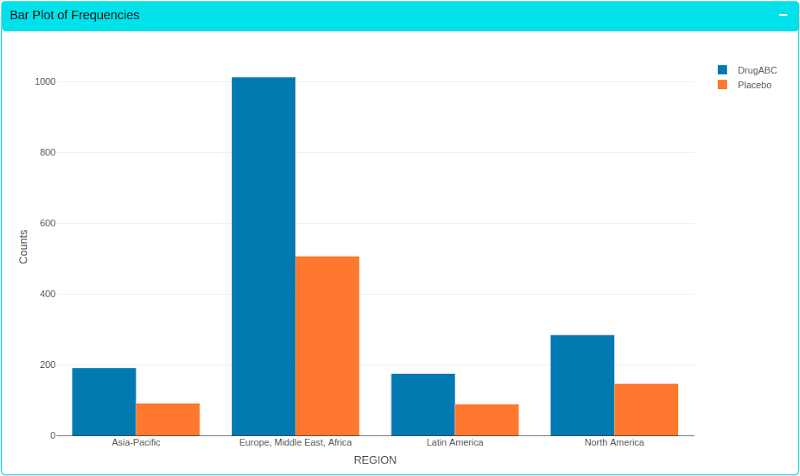
Instant Visualisation
Easily explore and visualize data, find hidden patterns and share with the teams instantly.

Study Dashboard
Dashboard shows up-to-date study information with patient flow, overview of safety and efficacy endpoints.
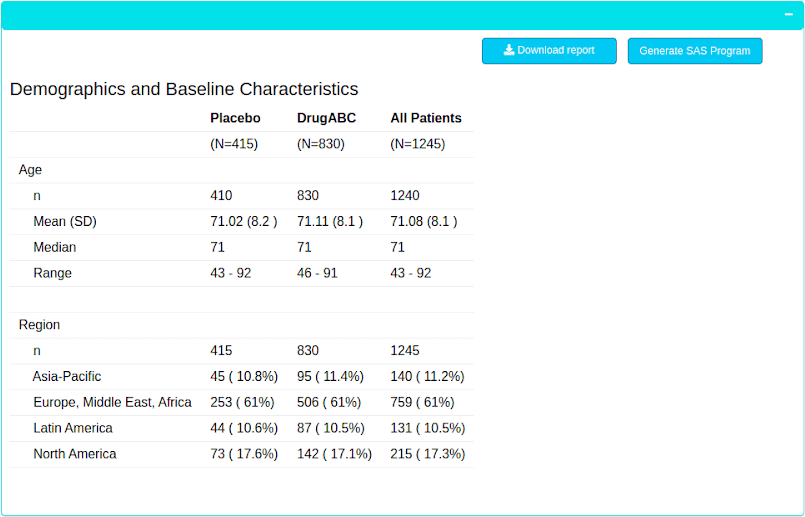
Report Generation
Any team member can interactively create custom reports, without writing any code. Submission ready SAS programs can be generated by clicking a button. Reports are also shareable across teams.
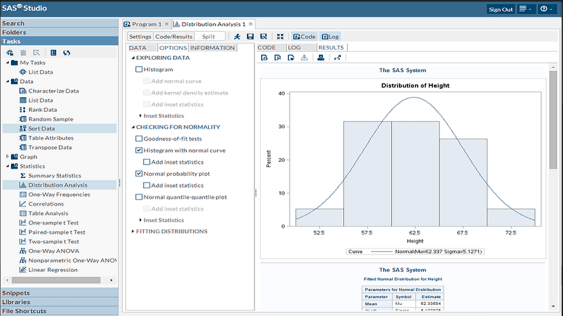
SAS® Studio Integration
MLytics Studio version control directly integrates with SAS® Studio version control. Programs generated through Autopilot, Guided Auto or Playground can be freely modified or customized in SAS® Studio, providing users complete control of the program functionality.
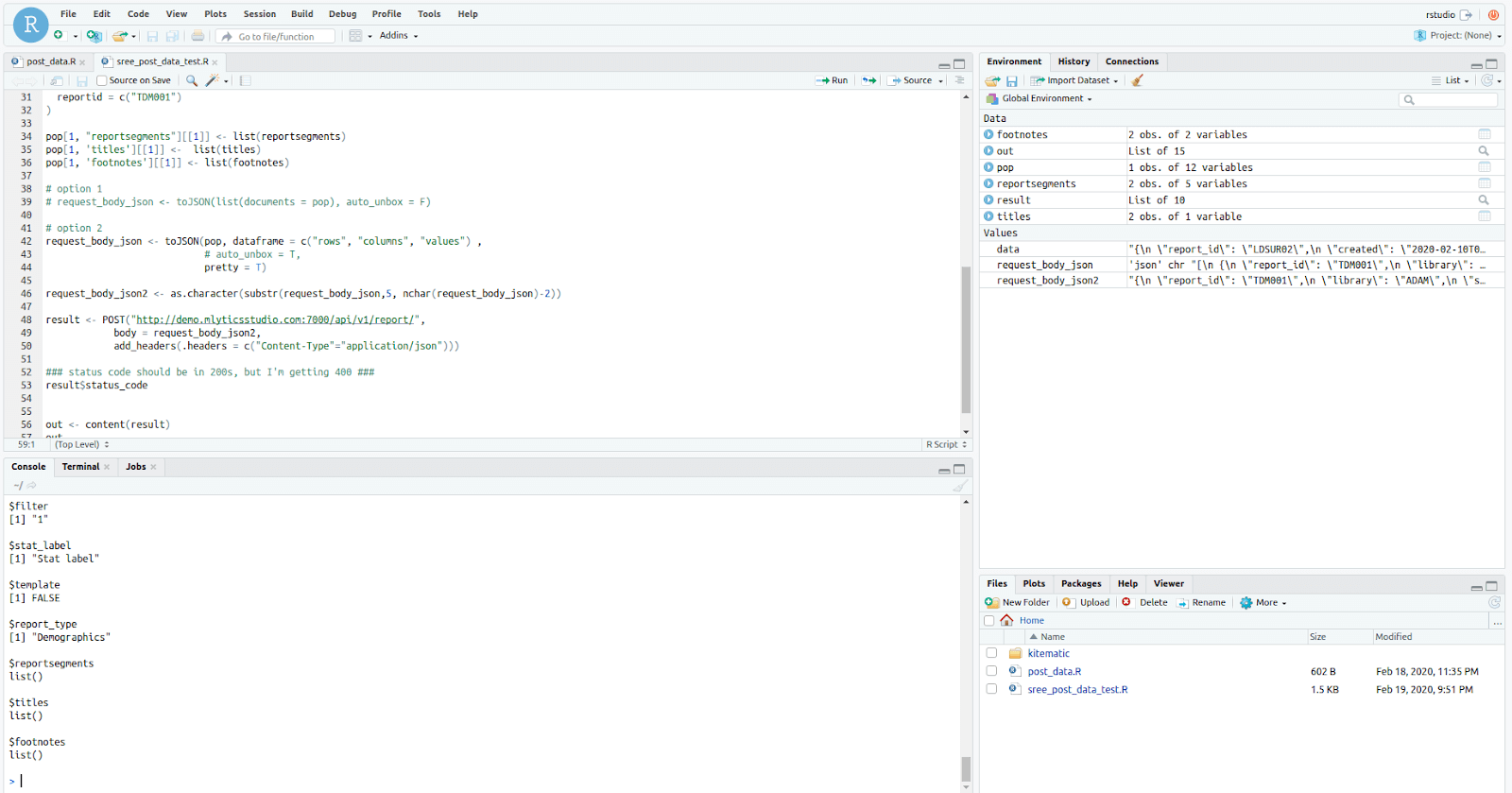
R Studio® Integration
Provides an integrated platform for leveraging the free and open-source software (FOSS), with its ever expanding array of useful packages for data exploration, analysis and visualization.
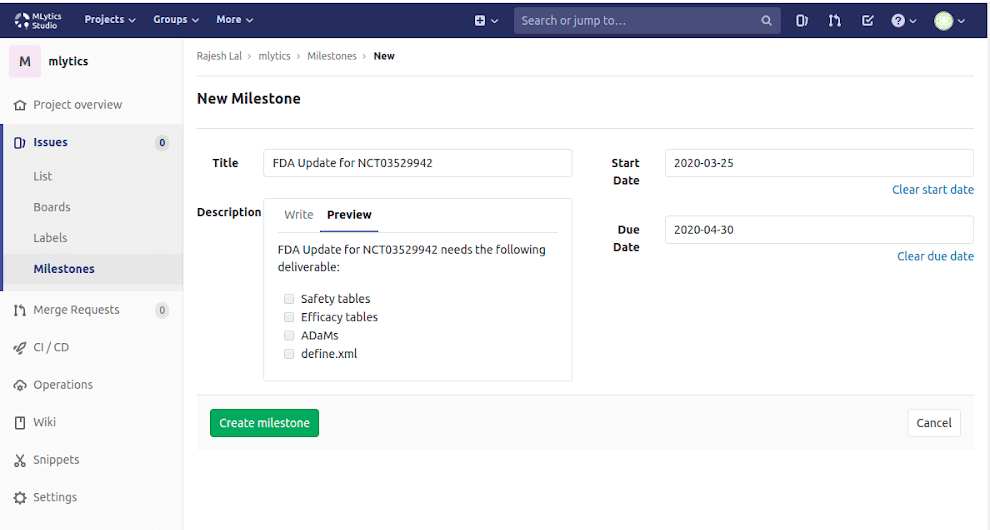
Project Planning
Allows project managers/leads to effectively manage teams and workloads. Easily create milestones, and organize tasks and deliverables to closure.
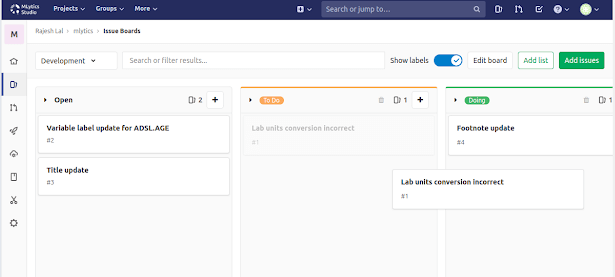
Project Tracking
Organize issues and updates among team members using Kanban boards and get the big picture of who is doing what. Easily update the status of your assignments by dragging the items between lists
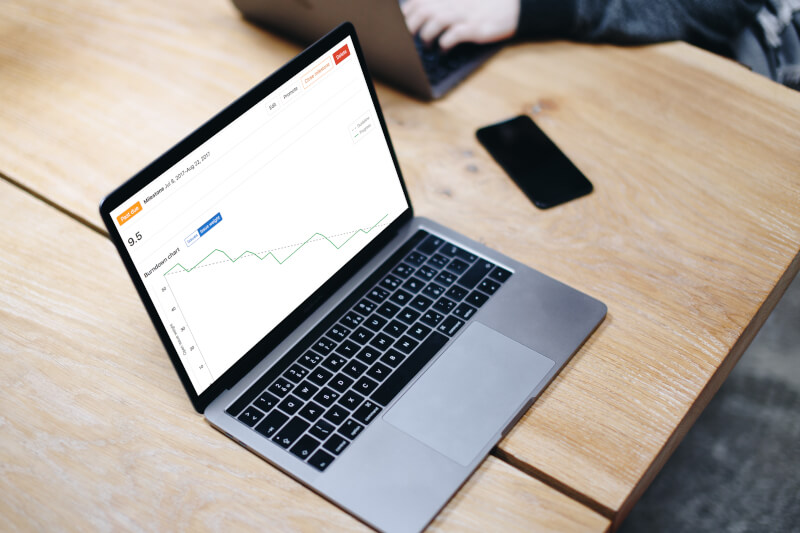
Burndown Chart
The chart displays planned vs. actual progress during a milestone. It provides a clear picture of how the team is progressing towards the goals.
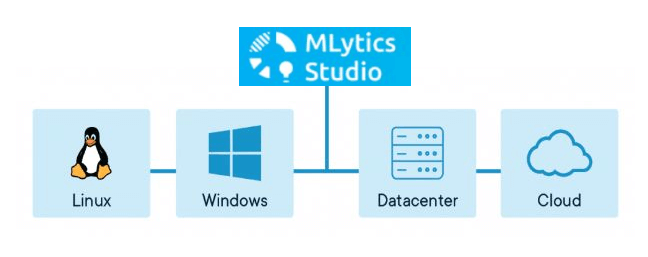
Scalability, compatibility, and security
MLytics Studio is scalable by design. By leveraging Docker and Kubernetes technologies, the application is scalable from 10s to 1000s of users. The application is compatible with all major platforms currently in use in the industry, works well with existing infrastructure, systems and processes and is secured by industry standard encryption.
Support
We’re Dedicated to Customer Success

Dedicated Customer Success Manager
Our best-in-class platform is backed by a best-in-class team committed to your success. MLytics Studio offers flexible packages, configured for your organization to accelerate your journey to success. Our experienced team works hand-in-hand with you, aligning business goals and objectives to the MLytics Studio Platform. Our commitment to your success begins on day one when we closely partner with you to deliver support and training. Provided to every new customer. Each onboarding project is managed through a dedicated customer success manager at MLytics, whose job it is to guide you seamlessly through setup and training.Contact Us
We look forward to being of service.
We endeavour to answer all questions within 48 hours.
MLytics Life Sciences LLC.
6749 S Westnedge Ste K205
Portage, MI - 49002.
